Ramblings: November 2023
17 December 2023
Below are collected Twitter ramblings for November 2023, with additional commentary interspersed as usual. This will likely be the last blog post of 2023. I wish everyone a relaxing and joyous holiday season in whatever way you choose to celebrate. This is also a natural time to reflect back on what went well and what could have gone better over the course of the year, and think about how you’ll make changes in the new year. I have some resolutions of my own in mind, but I’ll save those for another post. Without further ado…
This month’s photo of the month is a shot of the Verrazzano-Narrows Bridge taken from the New York City harbor, near sunset.
One thing I’ve seen too much of in the literature is the overselling of new concepts with only the barest of thought given to clinical translation. It’s particularly bad in flagship journals where people are trying to make a splash. I’ve read Science and Nature papers where giant, unwieldy peptides were described as translation-ready or “candidates” for development. This does a tremendous disservice to — and trivializes — the work of thousands of scientists in the drug discovery industry.
That kind of loose language might fly in a journal, but in drug discovery circles, the words “development candidate” have a precise, generally well-agreed-upon meaning among practitioners. Those words mean that the molecule has been put through its paces in in vitro and in vivo models, a full battery of PK and ADME assays looking for liabilities, and usually a dose range finding (DRF) toxicology study in a rodent and non-rodent species. The molecule is (predominantly) orally bioavailable in multiple preclinical species, and has an efficacious exposure predicted to lead to a reasonable efficacious dose in humans. Great attention has been paid to CMC (chemistry, manufacturing, and controls) concerns: how the compound will be safely manufactured on scale, understanding of its physical properties such as crystalline form, and the pharmaceutics work to understand compounding and formulation. This isn’t an exhaustive list, either, just enough to give a flavor.
Doing all of this stuff takes many years of work and isn’t a trivial exercise that you throw over the fence to me and my colleagues to dash off in a few weeks. It isn’t mere reduction to practice now that the hard intellectual lifting of having the brilliant idea in the first place is done and the paper is published. That translational effort is its own hard intellectual lift.
That’s not to say that radically new ideas can’t work. Take heterobifunctional degraders as a recent case-in-point. If you’ve heard Craig Crews lecture on this topic, you know that one of his main motivators to start a company was a clear-eyed realization that a lot of work had to be done by dedicated drug discovery professionals to turn this “chemical biology parlor trick” (his words) into clinical reality. I was fortunate to have a front row seat to many of the early years of that translational effort. But that’s the point: it was years of effort, and the outcome in those early days was far from certain.
Major props to our local meteorologists in Connecticut! I love that they don’t dumb things down for the audience all the time, and use phenomena that we encounter in the real world every day to teach scientific concepts.
The data for this example were loosely based on clinical JAK inhibitors. The main takeaway here is that you have to be careful translating a Kd in a biochemical assay to what’s going to happen in a cell, and ultimately, an organism. Biochemical assays are great for initial characterization of compounds. Their relative ease of running and high throughput means they often feature prominently in early tiers of a discovery screening cascade. They can, however, provide false comfort when it comes to understanding selectivity, or what drug concentrations will be needed to impact the target sufficiently to normalize a disease state in vivo. Cheng-Prusoff shifts from substrate competition, the need for high occupancy to normalize disease, and protein binding reducing free drug available to act on the target are three major headwinds that a biochemical assay will struggle to recapitulate.
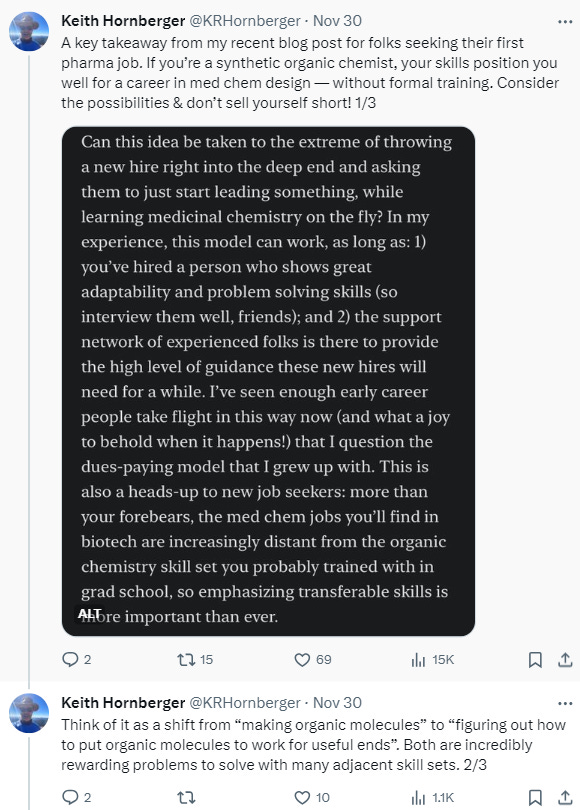
In the early days of your career, you have a lot to learn. It’s true in drug discovery generally, and medicinal chemistry more specifically, because so much of the training is on-the-job. It’s something that you have to live and breathe for years to really understand. I often struggle with how to answer when people ask me to recommend books or courses from which they can learn the practice of medicinal chemistry, because it’s all theory on the page. Furthermore, many published stories are sanitized for easier consumption and neglect to show all the blind alleys and wrong turns that the team invariably took along the way. It’s precisely those blind alleys — and how you recognize you’re in one and get out of it — that define the essence of medicinal chemistry. Like many things in life, we learn a lot more from our mistakes than our successes.
Eventually, though, you store up enough wisdom that you start to have some confidence in passing on what you’ve learned to others. It took me a solid 10 years to have enough experience to not feel like an amateur anymore, and 15 before I felt confident enough to teach. I’ve been blessed with an exceptional set of mentees over these last few years. Watching them grow and take flight, with the knowledge that you enabled that even a little, is gratifying. I won’t be doing this job forever. Building the success of the next generation is its own reward.
Behold what the LLMs have wrought. There are so many carbon copy accounts that spew the same AI-generated content, and I’m disgusted with it. There’s another subset of accounts that seem to be real people, but they lift content from other sites, sometimes verbatim, and pass it off as their own — hoping that nobody will do a Google search and notice. Remember why these accounts exist: they’re passive money-makers. They require a one-time effort to set up, and some minimal effort to curate. After that, the engagement and the likes — and the ads served — generate revenue for the account owner. Furthermore, no real new content is being created. These accounts cannot be called “creators” — they’re simply parasites living off the work of others.
These two go together. You would indeed have to drag me off the field to give up such a central concept. And it would definitely not be a match made in heaven to be working with people who didn’t think the same way.
Widely credited as the “father of toxicology” for this, his most famous saying, Paracelsus was also among the first to bring the science of chemistry into close contact with medicine. His theories were radical in their day and steeped in alchemy, but nonetheless had a grain of truth in them that grew with time.
The good-natured elbowing that goes on between medicinal chemists and process chemists is as old as the hills. Medicinal chemists are oft-accused by process chemists of designing shoddy syntheses without regard for yield or elegance — and there’s a note of truth in that, because yield and elegance are not our goal. We use organic synthesis as a means to an end — discovering a drug candidate molecule. Necessarily, hundreds or thousands of molecules will be made, tested, and discarded in a lead optimization campaign. Synthesis is thus oriented toward late-stage diversification to minimize the total number of synthetic operations. Yield is not a priority, and there’s an old joke that we only have two yields in med chem: enough and not enough. We do start to care about yield more though as we narrow in on that leading handful of compounds and need to start making 10s or 100s of grams. Medicinal chemists will typically retort to the process chemist that their job is easy, because they have the luxury of knowing which single compound they have to make, without having to worry about a thousand other dead ends. Or any biological or ADME data, where a single bad result among dozens can spell the demise of a promising compound.
It’s a single fraternity though, just with different chapters — and all the industrial chemists belong to it. The AI tech bros who are storming into drug discovery and treating it as another engineering problem to solve though? Good luck to them.
This is a key to good leadership, and runs counter to the skepticism engrained into scientists as part of their training. Skepticism taken to its extreme can become cynicism. You may have strong personal feelings on a subject, but there’s a time and a place to voice those feelings. In front of a team that you’re leading is not that place. Leaders set the tone for the whole team, and if you go low, you’re teaching your team that it’s okay for them to go low too. Go high and you’ll get a high performing team. Keep the focus on achieving outcomes that are within the team’s control, because that’s what empowers people. If you absolutely must: save the low for after work at the pub, preferably with people who are not on the team.
One of the things that makes medicinal chemistry so cool as a profession is that it’s highly interdisciplinary. There are many opportunities to learn new things adjacent to chemistry, and all of those things will make you better at your job. Furthermore, there’s only so much you can soak in from a classroom or a book. Eventually you have to have your own experiences doing the thing to get good at it. You also have to learn to lean into your failures — of which there will be many — because those are the most powerful learning moments.
Generality is important to a medicinal chemist and why we get accused of doing “boring chemistry”. Niche synthetic results that work well in a narrow context are generally not of high interest to us. We might have an intermediate that we want to diversify at the last step with cross-coupling chemistry to make 20 (or 100 — or 1000!) analogs, and we don’t know a priori which one of those many compounds will be the winner(s). If we have to choose between using a bunch of different reaction conditions, each carefully chosen to work well for a particular group of substrates, or blasting out the whole set regardless of yield with PdCl2dppf — we’re choosing the latter. We’ll sort the bodies out on the synthesis later once we know based on biological and ADME data which chemotypes are worth following up.
I’ve had some ringside seats to this phenomenon over the years, and no, I won’t be getting into the details. At least one such deal probably cost me my job in the restructuring that followed to help pay for the deal. Suffice to say that it’s important to listen to your own scientists. Yes, it’s fair to say that they may not be neutral, disinterested parties — but that doesn’t mean their opinions on such matters should be fully discounted in a wave of BD excitement. In my opinion, this is a case where it’s also important that senior leaders who will be making such decisions for an organization have at least some scientific background of their own and are not pure business-types.
If a team presents a series of results on a graph and draws pat conclusions, the first place my eye goes is to the outliers on the graph. I’m that guy who will ask the team for an explanation on the outliers, and I don’t like to settle for “I don’t know” or, worse still, tap dancing away from the problem. It may just be normal experimental variation, or the compounding effect of several small errors multiplied together — but that acknowledgement in and of itself teaches you something about the limitations of your experimental system. It lets you know where the limits of discrimination are, and is a ward against over-interpreting small differences as being meaningful. The highest performing teams, in my experience, are the ones that don’t shy away from these difficult questions, but rather focus some attention on understanding their data. Sometimes great discoveries are made in the exceptions to the rule.
I know it shouldn’t be the case that labs have “lucky” equipment. No less a Jedi than Obi-Wan Kenobi reminds us that “in my experience, there’s no such thing as luck.” But these little superstitions die hard. What lucky equipment do you have in your lab?
All of these go together. I think a lot of folks coming out of school from a synthetic organic chemistry background naturally gravitate toward process chemistry. It feels closer to home, and in some ways, it is. But ask any process chemist how much bearing the synthetic work they did in grad school has on the synthetic work they do in the plant, and they’ll happily disavow you of the notion that they’re all that similar.
Medicinal chemistry requires you to onboard a lot of knowledge that’s probably completely foreign to you as a synthetic chemist: the biology of human disease, pharmacology, ADME and PK, toxicology, and many other things. It can look a little daunting from the outside. But that’s exactly what makes it so cool! Few people in the world get the opportunity to work on such important problems at the intersection of so many disciplines.
It’s like being in a noisy street bazaar where the vendors in each stall are all speaking different languages and offering different things and haggling with you in different ways. Yes, it’s overwhelming when you’re first thrust into that scrum. Eventually, though, you begin to see the order in the chaos, and how the whole thing is like a living organism — and you’re a part of that! Moreover, the skills you’ve built as a synthetic organic chemist have equipped you more than you realize to navigate this environment. It’s a primary reason that such chemists are a preferred hiring pool for early career positions. You’re already a shrewd trader — you just need to learn the languages and wares of all the folks jostling around you in the bazaar.