Ramblings: September 2023
15 October 2023
Below are collected Twitter ramblings from September 2023, with the usual additional commentary interspersed.
This month’s photo of the month is from the white trail overlook at Sleeping Giant State Park in Hamden, CT. That’s Quinnipiac University, of the presidential polls, at the bottom of the cliffs. Sleeping Giant is a hiker’s paradise, and justifiably the busiest state park in the entire CT state park system.
These two go together. It’s impossible to pick just five impactful medicines out of the last century’s worth, but C&EN gave it their best shot. It was a great jumping-off point for further discussions around what else should be on the list, and the comments were chock-full of good suggestions. For my part, atorvastatin and lisinopril were no-brainers. Cardiovascular disease remains by far the #1 cause of death worldwide, so having representative meds on the list that lower cholesterol or blood pressure is logical. We can argue about which one(s) are our favorites, but these classes needed representation. Inhaled steroids for respiratory diseases also made my list, because COPD is the #3 cause of death behind heart disease and stroke. Fluoxetine (Prozac) was included because it was a flagship SSRI that began allowing us to tackle depression, OCD, and the like — moving psychiatric meds beyond the antipsychotics like chlorpromazine (Thorazine) on the C&EN list. Lastly, azathioprine and mercaptopurine were included as flagship antimetabolites from our friends Elion & Hitchings.
It really is the best and I won’t be taking any questions. Hundreds agreed too.
Hoo boy, this really did get published and it was a doozy. This paper was the catalyst for a couple of posts that followed, and ultimately a couple of tweetorials on receptor occupancy and drug promiscuity.
This chain of events is why publishing papers using tool compounds at 50-100 uM is dangerous. It sets off a less-than-virtuous cycle where bad conclusions drawn from bad chemical probes are propagated ever forward in the literature. Too many people lack the training in pharmacology to realize what’s wrong with this picture, and then they use these tools as if everything is fine.
I expected to get some reaction on the repo man. So here’s a little more on that. When I was in high school, I was very involved with the theater productions via the stage crew. One of our adult advisors had gone into technical theater professionally, but then left the biz and became a repo man. One night a couple of us got asked to make the rounds with him. We and our parents should have absolutely said no to this, but we did not because… I don’t know why, it was incredibly stupid. I rode around with the advisor dude in the repo truck. Yes, there was a large .38 caliber revolver in the glove box, and the cab is encased in bulletproof glass. Thankfully neither were needed.
Basically you just scout addresses and neighborhoods for vehicles on a list for repossession. If you find a make/model that’s right, you get out and read the VIN plate on the front of the dash. If it’s a match, the car will be up on the tow truck within 2 minutes or so. These guys work fast, because they’re often confronted (assaulted) by people who most definitely do not want their cars taken away from them, and time is of the essence. The rest of the guys were driving around in a personal vehicle making matches in the same way, but in their case the repossessions were via the motor credit companies (Ford Motor Credit, etc.)… which means when it’s time to repossess they literally send you the details to cut a key for the car. When a repossessor finds such a car, they will use a key to unlock the car, start the ignition, and drive it away to impound. Suffice to say this is incredibly dangerous, because again most car owners, even if behind on their payments, think that someone taking their car like this is stealing it. Our guys were chased by some pretty unhappy dudes on foot with baseball bats, more than once. I was happy to be in the bulletproof truck.
We only did this for one night, so it wasn’t much of a job. But it’s one hell of a story.
So many times. Interesting premise, reasonable looking chemical matter, and then… something creeps in. Maybe it begins with micromolar drug concentrations to see some functional effect in cells. Maybe that’s disconnected from on-target effects in a biochemical assay by an order of magnitude (or two). Trouble then escalates in the in vivo studies. Maybe the PK data was not included, or it was, but at doses / routes of administration that don’t match the PD and efficacy studies, leaving the reader with no way to decide if useful drug levels were achieved in vivo or not. Maybe plasma protein binding was omitted, so you have no sense of free drug levels in the studies. By the end, you’re holding your head in your hands and wondering why you spent time reading this thing which has become a train wreck. I read papers like this at least a couple times a week.
I think that naming and shaming is mostly bad form. There are plenty of reasons why a paper could have egregious violations of good practice that make the results uninterpretable. Sometimes it’s down to funding to run the necessary studies. Sometimes it’s down to folks not knowing what the necessary studies should be, and why. In any of those cases I want to bring those folks on side rather than alienate them, and all public shaming will do is burn bridges. I feel a better approach is to call out the problems generally, and then educate on what the correct approach is.
Iris was the one who re-did the superconductor prep at home, apparently in her kitchen. All the while reasoning through the flaws in the original prep and improving it with common sense. Alas the superconductor was not to be, but still a fun random connection to a niche Internet microcelebrity.
Specialization has allowed us to achieve some pretty amazing things in drug discovery, but we also run the risk of becoming so specialized that there’s no longer anyone left to see the big picture. It often falls to the medicinal chemist(s) on a discovery project to see that bigger picture, but that requires knowing enough biology, pharmacokinetics, pharmacology, toxicology, and a host of other things, to knit it all together. Or when we use those things, we often do so from a place of ignorance, blindly applying formulae without questioning whether or not they make sense in a given circumstance. Writ large, this is a societal problem. We have so much cool technology these days — way more than I ever could have dreamed of as a kid growing up in the 80s. But fewer and fewer people seem to know how any of this stuff works, or care to! It’s the lack of caring that concerns me the most. We should keep our curiosity alive and want to know how things work.
My opinion remains steadfast that the safety margins on acetaminophen are relatively narrow for an over-the-counter (OTC) drug. It’s reasonable to ask whether or not it should remain an OTC option when there are so many other pain reliever / fever reducer drugs that are OTC and don’t have the same complications. If the FDA can yank phenylephrine for lack of efficacy, they can yank acetaminophen for its narrow safety margin.
Some good and useful suggestions of how to defeat these publisher frames came up in the comments. The easiest is to just retype the URL, changing “epdf” to “pdf” and this has worked for me every time I’ve tried. I’m uncertain what publishers think they’re achieving with these reader frames, other than annoying their readers. Let’s face facts: nobody wants to just linger on the publisher’s website to read their journal articles. They’re reading articles from lots of publishers, and want to build personal libraries where they can aggregate and read and annotate all their literature together. Mendeley, ReadCube, and a host of other apps exist for exactly this purpose. I think publishers would better spend their time effectively integrating with the reading and annotation apps that are already out there rather than trying to keep folks lingering on their website. It doesn’t work anyway and just pisses people off.
Some explanations. I wouldn’t be a medicinal chemist if I didn’t pick palladium as a favorite element. I mean, carbon is an obvious choice too, but in terms of its impact on synthetic transformations, palladium is king. I’ve always had a soft spot for the Sandmeyer reaction and its ability to turn anilines into aryl halides, which opens up quite a universe of aromatic transformations that might otherwise be difficult. Azetidine is a good boi and I like him when you need a small and not-too-basic amine. Glutarimide: for obvious reasons, if you work on targeted protein degradation. I like the 100 mL rbfs with the wide neck because they’re easy to scrape product out of at the end. And ingenol: in-out isomerism is wild. I spent a chunk of my PhD trying and failing to build the core ring system. We never published the work because we didn’t get it anywhere, but it’s in my dissertation just the same.
This problem is perhaps magnified even more when considering things like heterobifunctional molecules and their ternary complexes. When inducing non-naturally occurring PPIs between two proteins, why would one think that a crystal structure snapshot is some kind of global minimum? It seems more likely in such cases that there are many local minima that are relatively close in energy as these two proteins that don’t normally interact slough against each other. X-ray crystal structures are a useful tool for sure, and have been used to great effect for optimization of chemical matter — even for degraders — but just the same: they’re not “the truth”, but rather “a truth”.
We’re so far into diminishing returns with BRD4 degraders. For those who don’t work in the targeted protein degradation space, BRD4 seems absurdly predisposed to being degraded. If you look at it funny, breathe on it, or the phase of the moon is just right… it degrades. Consequently, model degrader systems using BRD4 as the test bed are setting the lowest possible bar. Such systems are universally improved by looking at other things beyond BRD4. Better still would be to just not look at BRD4 at all. If you’re seeking to convince the world that your new E3 ligase ligand / degrader technology / whatever is the bee’s knees, BRD4 is not gonna get the job done.
It’s true that younger professionals (although I agree that the term “early career professionals” is probably better and more inclusive) have a specific set of needs and benefit from focused networking groups. In equal measure though, I’m too long in the tooth to belong to those groups, unless they want me to come out to be on their mid-career career panels. I’d like to see some social grouping of mid- and late-career professionals. The benefits of bonding and networking with peers are lifelong and don’t stop because you turned 35 or 40 or 10 years of career experience or hit some other arbitrary threshold.
Safety culture is important in industry. We take it seriously, and not just because lab accidents can expose a company to significant liability. It’s also the right thing to do, for everyone. Putting on a lab coat, or safety glasses, or gloves, or taking an extra minute to put a blast shield in front of something that could pop… these things should be core to our culture. The physical health and safety of our people should always be first.
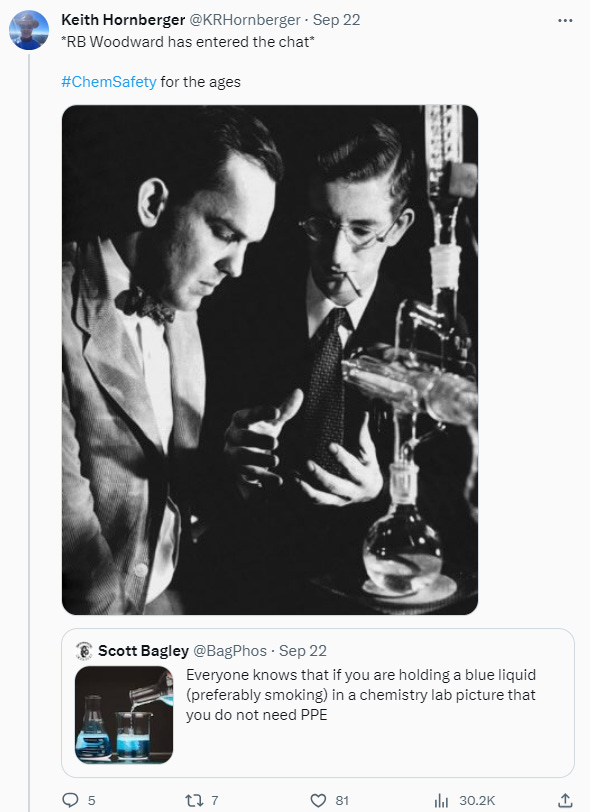
Chemistry labs are inherently dangerous places. It’s hopefully controlled, well-mitigated danger, but dangerous nonetheless. Everyone who’s been at this for a while has stories of accidents and near misses that they’ve either been the victim of or closely witnessed. Don’t wait until something tragic happens to build a safety culture. Woodward’s smoking in the lab with no PPE is a classic case of brilliant people doing stupid things.
I’ll drink to that! 🍻
It mystifies me how such unlikely coincidences occur. What are the odds of two people who know each other from Twitter, working for two different companies, flying halfway across the country to the same city on different business trips, and then landing in the same restaurant with dinner reservations within 30 minutes of each other? Surreal.