Tweetorial: One-Eyed Sheep
4 March 2023
I concede that I put a click-bait title on this tweetorial. The premise is so bizarre at first that you can’t help but wonder how the hell all of the dots got connected in the story. But really this story is two things. First, it’s a primer on the Hedgehog signaling pathway and inhibitors thereof as cancer therapeutics. Second, underneath the surface are some lessons on the non-linearity of scientific progress, where seemingly unconnected things become connected over a timespan of human lifetimes. This particular onion had a lot of layers, some of which weren’t accessible to us until the technology caught up to enable a deeper look.
This story begins as a problem with livestock. The early heroes here are from the US Department of Agriculture. A veterinarian and a chemist walk into a ranch… sounds like the beginning of a joke, but it’s the beginning of this tale. The USDA had been doing poisonous plant research since the 1890s, as livestock poisoning in various forms represents a serious economic problem. I can imagine some raised eyebrows in the office when they heard about lambs being born with one eye in Idaho.
Wayne Binns (the veterinarian) and Richard Keeler (the chemist) get the lion’s share of the credit in this early part of the story for figuring out which plant, and eventually which chemical compound, caused the teratogenicity. To them, and to ranchers, this was largely a solved problem by the early 1960s.
But it also speaks to where science was at the time, and how chemistry was leaps and bounds ahead of biology. Keeler was able to extractively isolate and then purify cyclopamine from Veratrum californicum, and correctly deduce its structure spectroscopically — because the tools were there to do that in 1960. Armed with the tools of organic synthesis, then in its golden age, Keeler continued doing SAR studies on cyclopamine into the 1970s. Meantime over in biology, the structure of DNA was less than a decade old. The entire field of molecular biology was embryonic. The tools were simply not there to understand the mechanism of action of cyclopamine at a molecular, genetic level.
By the late 1990s, that had changed. The second set of heroes in this story are Philip Beachy and the folks in his lab. Now kitted out with better tools that would have seemed like science fiction in the 1960s, they were doing mouse knockout studies and saw the same cyclopia and midline defects from deletion of Sonic hedgehog. What a tremendous flash of insight to find Binns and Keeler’s work on cyclopia from many decades prior and realize that cyclopamine could be directly acting on this pathway — and then proving it! No need to search for a chemical probe when somebody already found you one in nature. (Although the Beachy lab did later invest considerable effort in screening for synthetic antagonists.)
The concurrent discovery that Hedgehog pathway defects could cause cancer paved the way at last for the third and final set of heroes in this story: the legions of pharma industry scientists who developed fully synthetic Smoothened antagonists into drugs for basal cell carcinoma and other cancers. This is industry at its best, building on a foundation of years of basic research and then rapidly translating that work into a therapeutic agent. That kind of work rationally targeting a single gene/protein was really only coming to the fore at this time, and along with that a whole set of assay technologies to enable such rational drug discovery. Yet another case of right place, right time.
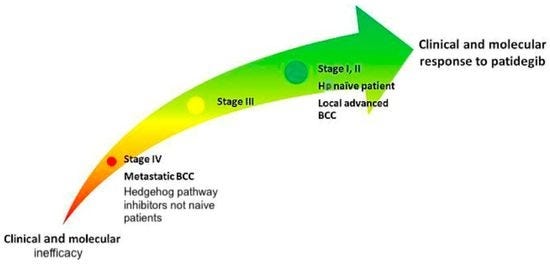
The oddest twist of all is the one that could yet bring this story full circle: if saridegib/patedegib, a semisynthetic analog of cyclopamine itself, eventually makes it to market, wouldn’t that be something? I can only imagine the folks at Infinity who decided to plant huge stands of Veratrum californicum to harvest kilos of cyclopamine must have felt some connection to the past, that they were walking the last mile in Binns & Keeler’s dusty boots.
Without further ado:













![The chemical structure of cyclopamine, IUPAC name: (2′R,3S,3′R,3′aS,6′S,6aS,6bS,7′aR,11aS,11bR)-3′,6′,10,11b-Tetramethyl-1,2,3,3′a,4,4′,5′,6,6′,6a,6b,7,7′,7′a,8,11,11a,11b-octadecahydro-3′H-spiro[benzo[a]fluorene-9,2′-furo[3,2-b]pyridin]-3-ol](https://substackcdn.com/image/fetch/$s_!aTpN!,w_600,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fpbs.substack.com%2Fmedia%2FFlTtojdX0AM8rWC.jpg)


















![Chemical structure of sonidegib; IUPAC name: N-[6-[(2S,6R)-2,6-Dimethylmorpholin-4-yl]pyridin-3-yl]-2-methyl-3-[4-(trifluoromethoxy)phenyl]benzamide](https://substackcdn.com/image/fetch/$s_!wI4a!,w_600,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fpbs.substack.com%2Fmedia%2FFlTtsJfXEAIh9ac.jpg)

![Chemical structure of gladegib; IUPAC name 1-[(2R,4R)-2-(1H-Benzimidazol-2-yl)-1-methyl-4-piperidinyl]-3-(4-cyanophenyl)urea](https://substackcdn.com/image/fetch/$s_!nm5p!,w_600,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fpbs.substack.com%2Fmedia%2FFlTtsz4XgAApApP.jpg)



![Chemical structure of saridegib; IUPAC name N-[(2S,3R,3′R,3aS,4′aR,6S,6′aR,6′bS,7aR,12′aS,12′bS)-3,6,11′,12′b-Tetramethyl-2′,3′,3a,4,4′,4′a,5,5′,6,6′,6′a,6′b,7,7′,7a,8′,10′,12′,12′a,12′b-icosahydro-1′H,3H-spiro[furo[3,2-b]pyridine-2,9′-naphtho[2,1-a]azulen]-3′-yl]methanesulfonamide](https://substackcdn.com/image/fetch/$s_!xE62!,w_600,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fpbs.substack.com%2Fmedia%2FFlTtuNaWYAAC9nG.jpg)