Tweetorial: Structural Alerts, Part Two
15 March 2022
Structural alerts as a topic lends itself to more than one installment. In the first go-round, I addressed redox cyclers. The most notorious in this category are nitro groups and quinones (and latent quinones!). After chucking those into the lake of fire and sulfur, it seems the appetite to discuss the medicinal chemist’s disdain for other functional groups was not sated - this was the clear poll winner for the next topic.
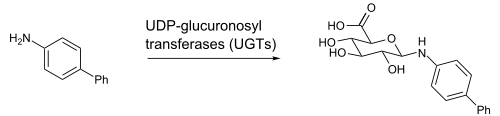
This time, I addressed anilines and phenols again, but from a different angle. These previously reared up in the context of redox cycling as latent quinones, and in the case of anilines, access to nitrenium ions and the like by oxidation. This time the focus was more on their potential metabolic liability as N- or O-glucuronides. That, in turn, led to a discussion of the most notorious of the glucuronides, the O-acyl glucuronides, which are derived from metabolism of carboxylic acids.
Much ink has been spilled in the literature about the potential tox liability of O-acyl glucuronides. Although they are a potential reason for concern, my opposition to carboxylic acids on these grounds is far from absolute. As I note in the thread, there are plenty of carboxlic acid-containing drugs, so not all carboxylic acids immediate give rise to toxic metabolites. Indeed, O-acyl glucuronides are the primary metabolite for many (most?) carboyxlic acids. Clearly not all are toxic, or toxic enough. To me, this is more of one of those things that you monitor for during safety studies - not necessarily a killer in its own right. (You’ll get wildly differing opinoins on this, though. I’ve known medicinal chemists who want to kill carboxylic acid-containing molecules that have demonstrated any ability to form an acyl glucuronide on sight.)
A bigger reason to be concerned about carboxylic acids is that they will be negatively charged at physiological pH (~7.4), and thus have a tough time getting across cell membranes. This negative impact on permeability can be a big obstacle to both gut absorption and cellular uptake at the site of action. In my experience anyway, that’s been the main barrier to progression of carboxylic acids. But again, not an insurmountable barrier, or there would be few statin drugs, among many others.
Anyway, here’s the thread. Happy reading!