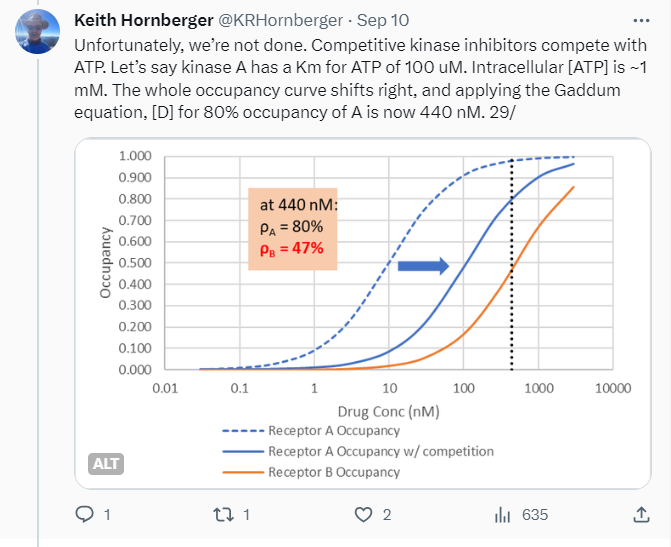
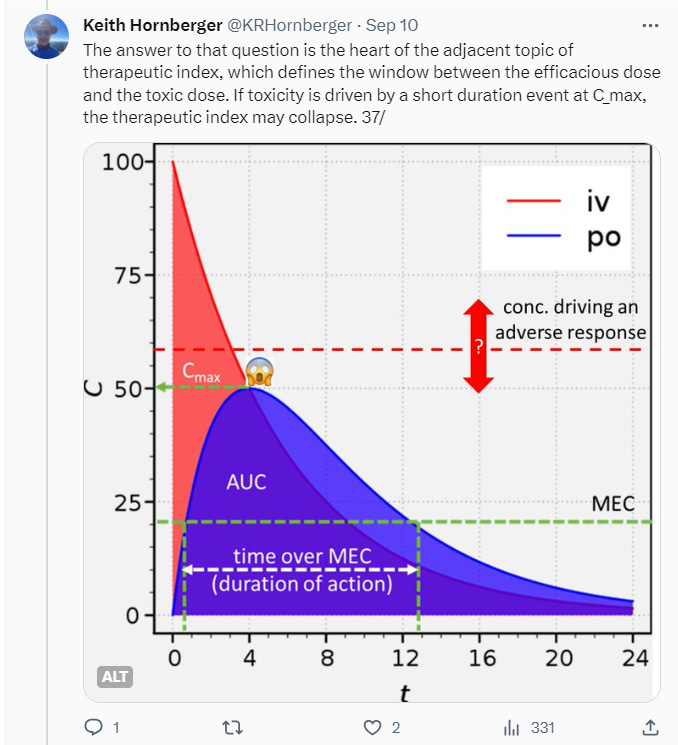
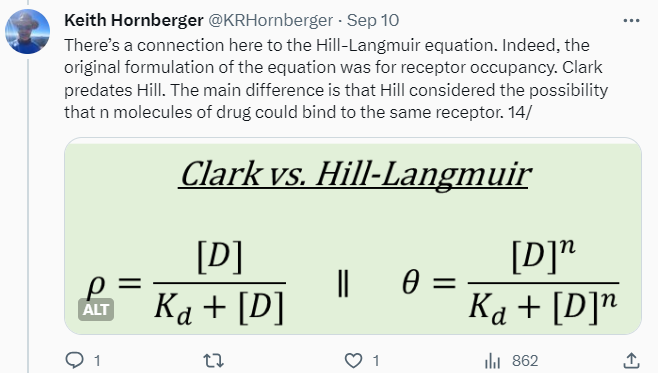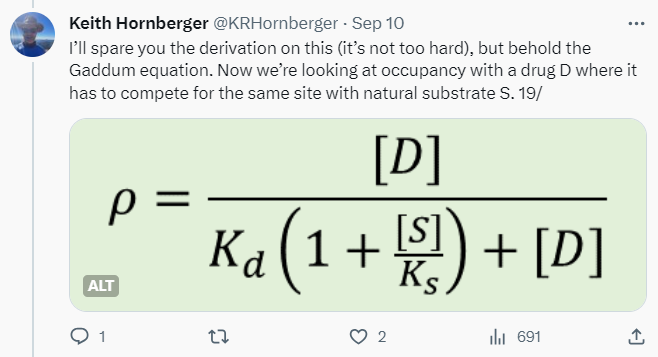After a long tweetorial dry spell this year, a double lightning bolt of inspiration struck me in September. These posts were triggered by reading a particularly egregious published example of a compound whose in vitro and in vivo profile was poorly characterized — most significantly by being used at absurdly high concentrations. Followed, of course, by the authors drawing sweeping conclusions about the biology of Intended Target X, when in fact no such conclusions were warranted.
Minimization of the importance of this problem in the practice of medicinal chemistry and allied disciplines, notably chemical biology, is a major contributor to ongoing literature pollution. A flawed study with poorly characterized chemical probes gets published, and then it’s often cited forevermore as gospel. Other labs will pick up that chemical tool, taking as a given that it’s a sufficiently potent and selective tool against the intended target (the “it’s published so it must be true” fallacy), and then draw further erroneous conclusions. And on, and on, and on.
This problem is addressable at several levels. Journal editors are the first line of defense and should be desk rejecting things where the chemical matter is insufficiently profiled to draw strong conclusions. Editors see a lot of manuscripts though, and not all editors may have the expertise themselves to spot problems. This is especially true in non-medicinal chemistry journals where a medicinal chemist may not be part of the editorial board.
The same thing can happen at the level of reviewers, who are the next line of defense. Whether due to lack of expertise or just being too overloaded to give manuscripts the thorough review they deserve, things still make it through the mesh of the sieve. One general review policy I’d like to see is that any manuscript containing new chemical probes should be reviewed by a card-carrying medicinal chemist.
I’m pleased to see more journals taking the right attitude on chemical probes. J. Med. Chem. pushed out a terrific editorial this year that talks about what proper in vivo characterization looks like. The Chemical Probes Portal remains a good and objective source on chemical probes, and there’s also a nice recent article getting into the nitty-gritty with proposed metrics from some of the same folks. I’d like to see journals subscribe more broadly to this kind of probe guidance — to the level of making authors run down a checklist prior to submission, and slapping warning labels on papers that don’t comport to the probe guidance. Ultimately journals taking a strong stance on this stuff is what’s needed to change behavior. When you can no longer get a Science, Nature, or Cell paper without proper characterization, and it becomes a prestige thing… then maybe people will care more.
There’s a lot of stick in that approach, but there are potential carrots too. The gentlest is doing what I do: providing some foundational education in pharmacology. Sometimes pharmacology is like the fly baseball that drops between a bunch of chemist and biologist and chemical biologist outfielders: they all think the other person’s got the ball.
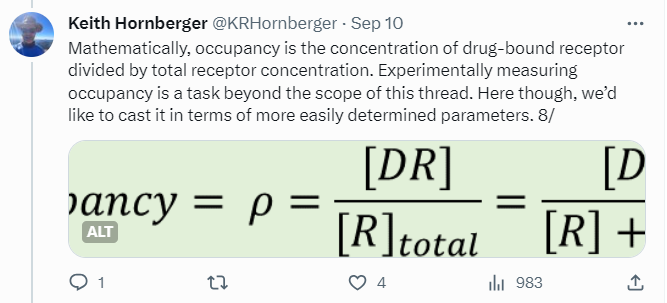
Tweetorials are never going to be the whole story, but they can be part of it. They’re designed to pique interest, to engage, and kick off further investigation of the topic on one’s own. My focus is on providing just enough theory to then talk about real-world consequences. In the receptor occupancy tweetorial, I review some foundational stuff like the Clark and Gaddum equations, most of which is easy for chemists who’ve survived general chemistry to understand — then delve into the disconnects between occupancy and efficacy, culminating in an all-too-plausible example where apparent “selectivity” blows up in your face.
Similarly, when talking about promiscuity, the focus is on building understanding of why micromolar (and above) binders run a significant risk of polypharmacology. Some of those wounds are self-inflicted with poor molecular design that can further promote polypharmacology, but eventually one reaches what I think of as a molecular discrimination limit — where the specificity of the system itself that you’re trying to interact with becomes insufficient. It’s not well appreciated that there are many families of proteins that have an evolved function that relies on nonspecific binding. I also deal here with the “ostrich problem”: measuring (often weak) binding to a target and then observing some cell-based functional effect that comports with one’s expectations of what modulating that target should do, and therefore concluding that one follows the other while looking no further. Unfortunately, in systems as complex as a cell, the hole in that logic is big enough to drive a truck through. There’s no substitute for selectivity screening, and when presented with a molecule that functions at concentrations approaching the molecular discrimination limit of the system, the burden of proof is high.
I’ve said nothing here about whether we should treat polypharmacology as a bug or a feature, and I’ll leave that topic alone for now. My main argument is that when seeking to ascribe functional outcomes to a specific target with chemical tools, you’d better be damn careful about how you do it. There is quite a debate these days about the value of intentional polypharmacology. There’s also a more recent analysis suggesting that target-based discovery is largely a failed paradigm, and we’d be better off going back to the days of phenotypic screening. While I think this latter paper has some significant methodological flaws despite its great pains to remain objective, it does raise an important topic for further discussion. There’s probably room for both in the end — this needn’t be an either/or.
Without further ado: